Newsletter Signup
Stay up to date on all the latest news from Boston.com
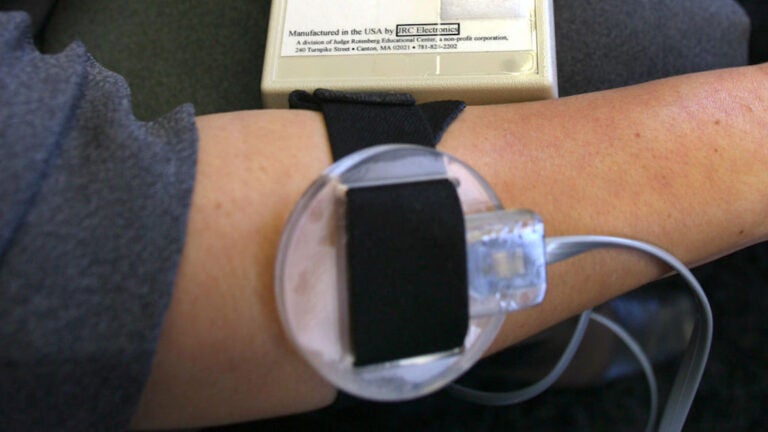
The Food and Drug Administration on Monday announced a new proposal to ban the use of devices that delivers electric shocks to curb behavioral problems, which are currently only used at a school in Massachusetts.
It is the second time the federal agency has proposed a ban on the devices, also known as ESDs, which deliver electric shocks through electrodes attached to the skin.
The Judge Rotenberg Education Center in Canton, a day and residential school that serves children and adults with disabilities, is the only facility in the country that uses the devices.
According to the FDA, the facility has about 50 individuals who “currently have a treatment plan that includes the use, or potential use, of an ESD.”
In a statement, Owen Faris, acting director of the Office of Product Evaluation and Quality in the FDA’s Center for Devices and Radiological Health, said the FDA has determined the devices “present an unreasonable and substantial risk of illness or injury.”
“These devices present a number of psychological risks including depression, anxiety, worsening of underlying symptoms, development of post-traumatic stress disorder, and physical risks such as pain, burns, and tissue damage,” Faris said. “The proposed rule, if finalized, will remove ESDs from the market, and the devices will no longer be considered legally marketed.”
In a statement obtained by Boston 25 News, the Judge Rotenberg Education Center said ESDs are “life-saving.”
“The Judge Rotenberg Educational Center (JRC) will continue to advocate for and, if necessary, litigate to preserve the court-approved, life-saving ESD treatment,” the center said. “FDA has once again made a decision to move forward with a ban of this treatment based on politics, not facts The D.C. Circuit Court of Appeals vacated a similar 2020 FDA rule banning the use of our treatment and we are confident that we will once again prevail on behalf of our clients and their families.”
The FDA’s first ban on the devices, put forward in 2020, was challenged in court and ultimately annulled. The agency said that since the decision, changes have been made to the Food, Drug, and Cosmetic Act that make it clear that it is the FDA’s purview to ban ESDs.
The agency said it is accepting public comment on the proposed ban until May 28, before a final rule is issued.
The Autistic Self Advocacy Network in a statement applauded the FDA’s latest proposed ban.
“Once the public comment period opens tomorrow, we need everyone to submit commits telling the FDA to #StopTheShock!” the organization wrote.

Dialynn Dwyer is a reporter and editor at Boston.com, covering breaking and local news across Boston and New England.
Stay up to date on all the latest news from Boston.com





Stay up to date with everything Boston. Receive the latest news and breaking updates, straight from our newsroom to your inbox.
Conversation
This discussion has ended. Please join elsewhere on Boston.com