Abstract
Chronic stress can have lasting adverse consequences in some individuals, yet others are resilient to the same stressor1,2. Susceptible and resilient individuals exhibit differences in the intrinsic properties of mesolimbic dopamine (DA) neurons after the stressful experience is over3,4,5,6,7,8. However, the causal links between DA, behaviour during stress and individual differences in resilience are unknown. Here we recorded behaviour in mice simultaneously with DA neuron activity in projections to the nucleus accumbens (NAc) (which signals reward9,10,11,12) and the tail striatum (TS) (which signals threat13,14,15,16) during social defeat. Supervised and unsupervised behavioural quantification revealed that during stress, resilient and susceptible mice use different behavioural strategies and have distinct activity patterns in DA terminals in the NAc (but not the TS). Neurally, resilient mice have greater activity near the aggressor, including at the onset of fighting back. Conversely, susceptible mice have greater activity at the offset of attacks and onset of fleeing. We also performed optogenetic stimulation of NAc-projecting DA neurons in open loop (randomly timed) during defeat or timed to specific behaviours using real-time behavioural classification. Both open-loop and fighting-back-timed activation promoted resilience and reorganized behaviour during defeat towards resilience-associated patterns. Together, these data provide a link between DA neural activity, resilience and resilience-associated behaviour during the experience of stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available on FigShare (https://figshare.com/projects/Behavioral_and_dopaminergic_signatures_of_resilience/141938). Source data are provided with this paper.
Code availability
All code is available on GitHub (https://github.com/lwillmore/QuantifyingDefeat).
References
Cohen, S., Janicki-Deverts, D. & Miller, G. E. Psychological stress and disease. J. Am. Med. Assoc. 298, 1685–1687 (2007).
van Praag, H. M., de Kloet, E. R. & van Os, J. in Stress, the Brain and Depression 225–259 (Cambridge Univ. Press, 2004).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Cao, J.-L. et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci. 30, 16453–16458 (2010).
Friedman, A. K. et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319 (2014).
Hultman, R. et al. Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell 173, 166–180.e14 (2018).
Peña, C. J. et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356, 1185–1188 (2017).
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Engelhard, B. et al. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 570, 509–513 (2019).
Parker, N. F. et al. Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat. Neurosci. 19, 845–854 (2016).
Ikemoto, S. & Panksepp, J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 31, 6–41 (1999).
Menegas, W., Akiti, K., Amo, R., Uchida, N. & Watabe-Uchida, M. Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat. Neurosci. 21, 1421–1430 (2018).
Menegas, W., Babayan, B. M., Uchida, N. & Watabe-Uchida, M. Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. eLife 6, e21886 (2017).
Akiti, K. et al. Striatal dopamine explains novelty-induced behavioral dynamics and individual variability in threat prediction. Neuron https://doi.org/10.1016/j.neuron.2022.08.022 (2022).
Tsutsui-Kimura, I., Uchida, N. & Watabe-Uchida, M. Dynamical management of potential threats regulated by dopamine and direct- and indirect-pathway neurons in the tail of the striatum. Preprint at bioRxiv https://doi.org/10.1101/2022.02.05.479267 (2022).
Larrieu, T. et al. Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress. Curr. Biol. 27, 2202–2210.e4 (2017).
Radwan, B., Jansen, G. & Chaudhury, D. Abnormal sleep signals vulnerability to chronic social defeat stress. Front. Neurosci. 14, 610655 (2020).
Nasca, C. et al. Multidimensional predictors of susceptibility and resilience to social defeat stress. Biol. Psychiatry 86, 483–491 (2019).
Wood, S. K., Walker, H. E., Valentino, R. J. & Bhatnagar, S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 151, 1795–1805 (2010).
LeClair, K. B. et al. Individual history of winning and hierarchy landscape influence stress susceptibility in mice. eLife 10, e71401 (2021).
Murra, D. et al. Characterizing the behavioral and neuroendocrine features of susceptibility and resilience to social stress. Neurobiol. Stress 17, 100437 (2022).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Cox, J. & Witten, I. B. Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci. 20, 482–494 (2019).
Steinberg, E. E. et al. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 16, 966–973 (2013).
Saunders, B. T., Richard, J. M., Margolis, E. B. & Janak, P. H. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 21, 1072–1083 (2018).
Adamantidis, A. R. et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 31, 10829–10835 (2011).
Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019).
Golden, S. A., Covington, H. E. 3rd, Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191 (2011).
Serchov, T., van Calker, D. & Biber, K. Sucrose preference test to measure anhedonic behaviour in mice. Bio-protocol 6, e1958 (2016).
Kudryavtseva, N. N., Bakshtanovskaya, I. V. & Koryakina, L. A. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 38, 315–320 (1991).
Muir, J. et al. In vivo fiber photometry reveals signature of future stress susceptibility in nucleus accumbens. Neuropsychopharmacology 43, 255–263 (2018).
Motta, S. C. et al. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc. Natl Acad. Sci. USA 106, 4870–4875 (2009).
Lee, W., Fu, J., Bouwman, N., Farago, P. & Curley, J. P. Temporal microstructure of dyadic social behavior during relationship formation in mice. PLoS ONE 14, e0220596 (2019).
Wesson, D. W. Sniffing behavior communicates social hierarchy. Curr. Biol. 23, 575–580 (2013).
Williams, A. V. et al. Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology 45, 1423–1430 (2020).
Harris, A. Z. et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43, 1276–1283 (2018).
Takahashi, A. et al. Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 7, 12838 (2017).
Yohn, C. N. et al. Chronic non-discriminatory social defeat is an effective chronic stress paradigm for both male and female mice. Neuropsychopharmacology 44, 2220–2229 (2019).
Lee, H. et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014).
Schmack, K., Bosc, M., Ott, T., Sturgill, J. F. & Kepecs, A. Striatal dopamine mediates hallucination-like perception in mice. Science 372, eabf4740 (2021).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Dai, B. et al. Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Rep. 40, 111246 (2022).
Hamid, A. A. et al. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 19, 117–126 (2016).
Cai, L. X. et al. Distinct signals in medial and lateral VTA dopamine neurons modulate fear extinction at different times. eLife 9, e54936 (2020).
Wang, D. V. & Tsien, J. Z. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS ONE 6, e17047 (2011).
Dias, C. et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 516, 51–55 (2014).
Bagot, R. C. et al. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron 90, 969–983 (2016).
Montague, P. R., Dayan, P. & Sejnowski, T. J. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 16, 1936–1947 (1996).
Datta, S. R., Anderson, D. J., Branson, K., Perona, P. & Leifer, A. Computational neuroethology: a call to action. Neuron 104, 11–24 (2019).
Lammel, S. et al. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 85, 429–438 (2015).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e22 (2018).
Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L. & Tank, D. W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57 (2007).
Lindzey, G., Winston, H. & Manosevitz, M. Social dominance in inbred mouse strains. Nature 191, 474–476 (1961).
Fan, Z. et al. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 14, 819–831 (2019).
Byers, S. L., Wiles, M. V., Dunn, S. L. & Taft, R. A. Mouse estrous cycle identification tool and images. PLoS ONE 7, e35538 (2012).
Friard, O. & Gamba, M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330 (2016).
Fink, A. J. P., Axel, R. & Schoonover, C. E. A virtual burrow assay for head–fixed mice measures habituation, discrimination, exploration and avoidance without training. eLife 8, e45658 (2019).
Berman, G. J., Choi, D. M., Bialek, W. & Shaevitz, J. W. Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11, 20140672 (2014).
Choi, J. Y. et al. A comparison of dopaminergic and cholinergic populations reveals unique contributions of VTA dopamine neurons to short-term memory. Cell Rep. 33, 108492 (2020).
Pisano, T. J. et al. Homologous organization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep. 36, 109721 (2021).
Renier, N. et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802 (2016).
Chon, U., Vanselow, D. J., Cheng, K. C. & Kim, Y. Enhanced and unified anatomical labeling for a common mouse brain atlas. Nat. Commun. 10, 5067 (2019).
Acknowledgements
We thank C. Peña, J. Shaevitz and members of the Witten and Falkner laboratories for useful discussions; R. Cho for comments on a previous version of the manuscript; A. Zhukovskaya and A. Minerva for assistance with rebuttal materials; and staff at the PNI Viral Core Facility for reagents and the PNI Brain Registration and Histology Core Facility for assistance with histology. Funding was from NIH T32MH065214 (to L.W.), NSF GRFP DGE-2039656 (to L.W.), NIMH DP2MH126375 (to A.L.F.), NIH R01MH126035 (to A.L.F.), ARO W911NF1710554 (to I.B.W.), NIH R01 DA047869 (to I.B.W.), NYSCF (to A.L.F. and I.B.W.), SCGB (to A.L.F. and I.B.W.), Klingenstein Foundation (to A.L.F.) and an Alfred P. Sloan Fellowship (to A.L.F.). A.L.F. and I.B.W. are New York Stem Cell Foundation Robertson Investigators.
Author information
Authors and Affiliations
Contributions
L.W., I.B.W. and A.L.F. conceived the project. L.W. and C.C. collected data. L.W. and J.Y. analysed data. A.L.F. and I.B.W. advised on the data analysis. L.W., I.B.W. and A.L.F. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Anna Beyeler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
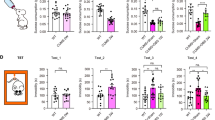
Extended Data Fig. 1 Pretests do not predict susceptibility while additional post-hoc tests confirm resilient and susceptible phenotypes.
a, Timeline of behaviors for mice undergoing behavioral and neural recordings across chronic social defeat stress. b, In the social interaction test, social interaction ratio and time (N = 22 controls, 13 susceptible, 19 resilient males in b–k; 1-way ANOVA for social interaction ratio with mouse category as factors, F(51,2) = 7.015, p = 0.002, t-Test control vs susceptible p = 0.001, resilient vs susceptible p = 0.009, control vs resilient p = 0.646; 1-way ANOVA for social interaction social interaction time with mouse category as factors, F(51,2) = 42.9, p = 1.18E-11, t-Test control vs susceptible p = 1.6E-9, resilient vs susceptible p = 1.6E-9, control vs resilient p = 0.9). In social interaction test, object interaction ratio and time (1-way ANOVA object interaction ratio with mouse category as factors, F(51,2) = 9.442, p = 3.3E-4; t-Test control vs susceptible p = 0.003, resilient vs susceptible p = 0.001, control vs resilient p = 0.5; 1-way ANOVA for object interaction time with mouse category as factors, F(51,2) = 27.26, p = 8.86E-9; t-test control vs susceptible p = 0.003, resilient vs susceptible p = 0.001, control vs resilient p = 0.6). c, Fraction of sucrose over total volume consumed in a 2-bottle sucrose preference test (1-way ANOVA for sucrose preference with mouse category as factors, F(51,2) = 3.21, p = 0.048; t-Test control vs susceptible p = 0.039, resilient vs susceptible p = 0.024, control vs resilient p = 0.9). d, Relationship between sucrose preference and SI time (2-sided pearson correlation coefficient R = 0.29, p = 0.03). e, Change in weight across defeat (top), absolute weight (bottom), mean±SEM plotted (t-test for difference of means between resilient vs. susceptible and resilient vs. control groups, green stars indicate resilient vs susceptible and black indicate resilient vs control significance, see Supplementary Table 1 for statistical values). f, Relationship between SI time and the change in weight during defeat (2-sided GEE, grouped by mouse, Z = 2.01, p = 0.045). g, Number of crossings between chambers of a 2-chamber arena (1-way ANOVA for crossings with stimulation groups as factors, F(51,2) = 5.77 p = 0.006; post-hoc t-Test susceptible vs control p = 0.011 and resilient vs control p = 0.008). h, Percent of time immobile (speed < 1 cm/s) during exploration of a 2-chamber arena (1-way ANOVA for crossings with stimulation groups as factors, F(51,2) = 3.16 p = 0.05; post-hoc t-Test susceptible vs control p = 0.032 and resilient vs control p = 0.071). i, Schematic of tube test for social hierarchy (top), example of tube test wins for a homecage of mice (colored by individual) measured across 7 days. j, No relationship between dominance (percentage of wins in the last 3 days of the tube test) and social interaction time in the social interaction test (N = 22 controls, 13 susceptible, 19 resilient; 2-sided Pearson’s correlation coefficient R = −0.038, p = 0.78). k, Summary of j (1-way ANOVA for social interaction social interaction time with mouse category as factors, F(51,2) = 0.342, p = 0.712). l, Time spent in proximity with a male of the same strain (C57/BL6), in a freely moving environment (2-way mixed ANOVA with resilience group and time point as factors: group x time F(64,2) = 0.319, p = 0.728) m, Time spent in proximity with a female of the same strain (C57), in a freely moving environment. (2-way mixed ANOVA with resilience group and time point as factors: group x time F(64,2) = 0.189, p = 0.828) n, Difference in time spent with a male or female social target after vs before defeat, mean±SEM plotted. N = 11 control, 8 susceptible, and 16 resilient males in l–n. Across panels, *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 2 Evolution of defeat behaviors across defeat days.
Amount of time spent in classified behaviors across days, mean±SEM plotted (2-sided GEE for time attacked: effect of resilience group Z = 0.23, p = 0.76, effect of day Z = −0.613, p = 0.54; 2-sided GEE for time investigated Z = −0.43, p = 0.66, effect of day Z = 4.01, p = 6.2E-5; 2-sided GEE for fighting while attacked: effect of resilience group Z = 2.09, p = 0.037, effect of day Z = 0.02, p = 0.98, 2-sided GEE for fleeing while attacked: effect of resilience group Z = −1.48, p = 0.14, effect of day t = 1.76, p = 0.078, N = 19 resilient and 13 susceptible mice. *** indicates p < 0.001).
Extended Data Fig. 3 t-SNE clusters of social postures represent discrete behaviors, some of which are preferentially occupied by resilient or susceptible mice.
a, Average raw feature value within each t-SNE cluster (scale in the full range of each variable, Str: Stressed mouse, Agg: Aggressor mouse). b, Average t-SNE cluster occupancies for each individual, split by susceptible and resilient groups (2-sample, 2-sided T-test *p < 0.05; 2-sample Levene Test ┼p < 0.05, N = 13 susceptible males and 19 resilient, see Supplementary Table 1 for statistical values). c, Density of behaviors annotated with supervised classifiers within t-SNE space, bold font indicating clusters that differentiate resilient and susceptible mice (Fig. 1o–r).
Extended Data Fig. 4 Behavioral and physiological differences across defeated females.
a, Distribution of social interaction (SI) times in the post-hoc SI test (see Fig. 1b) in unstressed control and stressed females split into resilient and susceptible groups (N = 4 controls, 4 susceptible, 4 resilient females, mean±SEM plotted). b, Change in weight across defeat by females in control, resilient, or susceptible groups, mean±SEM plotted. c, Fraction of sucrose over total volume consumed in a 2-bottle sucrose preference test, mean±SEM plotted. d, Number of entries into the open arms of an elevated plus maze, mean±SEM plotted. e, Time to consume a yogurt treat in novelty suppressed feeding assay, mean±SEM plotted. f, Time spent by individual mice in each behavior of interest (2-sided GEE: being attacked p = 0.76; being investigated p = 0.26; fighting while attacked, p = 0.16; fleeing while attacked p = 0.20; means plotted with 30th to 70th percentile error bands across the 10 defeat days; N.S., not significant). g, Difference in time males and females spent fighting back while attacked during defeat (2-sample t-Test, t = −7.45, p = 6.2E-9, N = 32 males, 8 females, mean±SEM plotted, ***p < 0.001).
Extended Data Fig. 5 Simultaneous fast scan cyclic voltammetry and fiber photometry calcium recordings in the TS.
a, Schematic for recording dopamine release and calcium fluorescence in the tail of the striatum (TS). b, Example 3-D color plot from a single recording site, where the medial forebrain bundle (MFB) was stimulated with 24 pulses at 60 Hz at time 0 (average of 5 trials shown). Inset shows the voltammogram at 0.5s after stimulation (white dotted line in color plot), which shows oxidation and reduction peaks consistent with the profile of dopamine. c, Time course of simultaneously measured calcium fluorescence and dopamine current (average over the same 5 trials shown in b). d, Calcium fluorescence and dopamine concentration peaks increase together with the number of pulses of stimulation delivered to the MFB (mean±SEM plotted, N = 6 recording sites). VTA-NAc data obtained with permission from Parker et al 2016 (N = 4 recording sites).
Extended Data Fig. 6 DAT::GCaMP6f responses to air puff, food reward, and defeat are similar in males and females.
a, In males, DAT::GCaMP6f aligned to air puff onset, averaged within and across mice (N = 19 males, paired t-Test for average activity after vs before puff, TS: t = 4.61, p = 2.2E-4; NAc: t = −3.1, p = 0.0062). b, Same as a but in females (N = 12 females, paired t-Test for average activity after vs before puff, TS: t = 3.05, p = 0.011; NAc: t = −1.2, p = 0.25). c, In males, DAT::GCaMP6f aligned to palatable food reward approach, averaged within and across mice (N = 18 males, paired t-Test for average activity after vs before approach, TS: t = 1.63, p = 0.12; NAc: t = 4.31, p = 4.7E-4). d, Same as c but in females (N = 11 females, paired t-Test for average activity after vs before approach, TS: t = 1.74, p = 0.11; NAc: t = 6.67, p = 5.6E-5). e, Average proximity onset- and offset-aligned TS(DAT::GCaMP6f) across stressed males (top) and females (bottom). f, TS(DAT::GCaMP6f) in the 0.5s at the onset or offset of social encounters (gray shaded zones in e) in males (top) and females (bottom) from resilient and susceptible groups across the 10 days of defeat. Solid line shows mean across mice and shaded region shows standard error. (2-sided GEE regression for male onset activity: main effect of day Z = 5.71, p = 1.1E-8; resilience Z = −0.83, p = 0.407; 2-sided GEE regression for male offset activity: main effect of day Z = 0.45, p = 0.65; resilience Z = 0.107, p = 0.917; 2-sided GEE regression for female onset activity: main effect of day Z = 0.673, p = 0.501; resilience Z = −1.912, p = 0.054; 2-sided GEE regression for female offset activity: main effect of day Z = −1.07, p = 0.28; resilience Z = 3.033, p = 0.003; interaction between day and resilience Z = 2.326, p = 0.02). g, Same as e for NAc(DAT::GCaMP6f). h, Same as f for NAc(DAT::GCaMP6f) (2-sided GEE regression for male onset activity: main effect of day Z = −1.328, p = 0.184; resilience Z = 1.98, p = 0.048; 2-sided GEE regression for male offset activity: main effect of day Z = 4.966, p = 6.8E-7; resilience Z = −2.06, p = 0.040; 2-sided GEE regression for female onset activity: main effect of day Z = 1.369, p = 0.171; resilience Z = 1.369, p = 0.402; 2-sided GEE regression for female offset activity: main effect of day Z = −0.074, p = 0.941; resilience Z = −1.29, p = 0.199). Across panels, *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 7 In males and females, relationships DAT::GCaMP6f and social zone or object zone entry or exit during the SI test.
a, Same data as in Fig. 2i, separated by male (left, N = 17) and female mice (right, N = 7). (2-sided pearson correlations between GCaMP and social interaction test social interaction (SI) time: Male entry R = 0.53, p = 0.03; male exit R = −0.15, p = 0.57; female entry R = 0.51, p = 0.24; female exit R = −0.73, p = 0.06). b, Average social interaction zone entry- and exit-aligned TS(DAT::GCaMP6f) across stressed animals (mean plotted with standard error, N = 6 susceptible males, 4 susceptible females, 10 resilient males, 3 resilient females in b–g). c, Relationship between individuals’ SI time and average TS(DAT::GCaMP6f) in the 1s after entry into the social interaction zone (left, 2-sided pearson correlation, R = −0.32 p = 0.13) and in the 1s after exit from the social interaction zone (right, 2-sided pearson correlation, R = 0.25, p = 0.23). d, Average object zone entry- and exit-aligned TS(DAT::GCaMP6f) across stressed animals. e, Relationship between individuals’ SI test interaction time and average TS(DAT::GCaMP6f) in the 1s after entry into and exit from the object interaction zone (entry: 2-sided earson correlation, R = 0.06 p = 0.8; exit: 2-sided pearson correlation, R = 0.34, p = 0.107). f, Same as in d for NAc(DAT::GCaMP6f). g, Relationship between individuals’ SI test interaction time and average NAc(DAT::GCaMP6f) in the 1s surrounding entry into and exit from the object interaction zone (entry: 2-sided pearson correlation, R = 0.15 p = 0.47; exit: 2-sided pearson correlation, R = 0.15, p = 0.47). In b,d,f: mean±SEM plotted.
Extended Data Fig. 8 Fiber location for photometry recording and behavior encoding by the TS does not predict resilience in males.
a, Relationship between average time spent engaging in each behavior and kernel weight for NAc(DAT::GCaMP6f) encoded by corresponding behavioral events (2-sided pearson correlation, *p < 0.05, N = 19 males, purple designating resilient animals, green showing susceptible, see Supplementary Table 1 for statistical values). b, Anatomical locations of photometry fiber tips targeted to the nucleus accumbens (NAc) and tail of the striatum (TS), overlaid on regions defined by the Paxinos Atlas (scale bar 1mm, Paxinos splits TS into subregions of the caudal caudoputamen). Animals omitted on the basis of poor signal shown in red, or omitted on the basis of poor targeting shown in magenta, included animals shown in black. c, Lack of relationship between location of recording fiber tips and SI time. d, Average kernel weights for behaviors encoding NAc(DAT::GCaMP6f) in mice grouped by fiber location in the NAc (p-values shown for 2-sided t-test for difference in means in average activity from 0.5 to 1.5s following the behavior event, see Supplementary Table 1 for statistical values). e, Average kernel weights for behaviors encoding TS(DAT::GCaMP6f) in mice grouped by susceptibility (p-values shown for 2-sided t-test for difference in means in average activity from 0.5 to 1.5s following the behavior event, N = 7 susceptible (green) and 12 resilient (purple) mice, see Supplementary Table 1 for statistical values). Across panels, *p < 0.05, **p < 0.01.
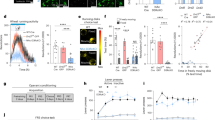
Extended Data Fig. 9 Additional characterization of optogenetic manipulation of DA neural activity during defeat.
a, Arena schematic and example stimulation session trace from a 2-chamber real-time place preference assay. 20-Hz laser stimulation was delivered whenever a mouse’s centroid was in the “laser side” of the chamber, sides counterbalanced across mice (top). Preference for the laser-stimulation side of a chamber in mice expressing or not expressing excitatory opsin. No stimulation was delivered in the baseline condition (bottom). b, Animals receiving fight-back-triggered optogenetic activation expressed either ChRmine or ChR2 and were delivered 20 Hz laser stimulation with the following parameters: ChRmine mice received green laser stimulation in sets of 3 pulses without pause for the duration of fighting bouts while ChR2 mice received blue laser stimulation in sets of 10 pulses with a 0.5s pause between pulse sets for the duration of fighting bouts. c, Example of a defeat session with laser light delivery triggered on fighting of the closed-loop mouse and simultaneously delivered to his open-loop neighbor, bottom inset is a 10s segment. d, Statistics across fight-back-triggered stimulation: Distribution of latency to trigger stimulation from capture of a fighting video frame, with a median of 100ms (left). Distribution of durations of stimulation bouts, 94% of which were less than 1s (middle). Distribution of the amount of defeat time in which light was delivered (mean is 2.99% of the defeat session) (right). e, Attack-offset aligned laser stimulation during escape-triggered activation. f, In escape-triggered activation, distribution of the amount of defeat time in which light was delivered (mean is 0.75% of the defeat session). g, Time spent in random forest labeled fighting behavior early (days 1,2) versus late (days 9,10) in defeat, mean±SEM plotted. (Paired 2-sided t-Tests: 2-way FDR corrected: open-loop t = 0.38 p = 0.71, no opsin t = −0.41 p = 0.71; 2-way FDR corrected: escape-triggered t = −2.33 p = 0.10, no opsin t = −1.14 p = 0.34). h, Average expression of random forest labeled fleeing behavior early (days 1,2) versus late (days 9,10) in defeat, mean±SEM plotted. (Paired 2-sided t-Tests: 3-way FDR corrected: open-loop t = 1.12 p = 0.42, fight-triggered t = −0.29 p = 0.78, no opsin t = −1.49 p = 0.42). i, Percent of time immobile (speed < 1 cm/s) during exploration of a 2-chamber arena (2-sided t-Test, difference in mean immobility between open-loop (N = 14 males) and no opsin control (N = 14 males) group, mean±SEM plotted: t = −1.57, p = 0.12). j, Same as i for fight-back-triggered (N = 16 males) and no opsin (N = 14 males) controls, mean±SEM plotted (2-sided t-Test, t = −3.05, p = 0.005). k, Same as i for escape-triggered (N = 8 males) and control (N = 4 males) groups, mean±SEM plotted (2-sided t-Test, t = −0.80, p = 0.39). l, Schematic of inhibition of dopaminergic cell bodies in the VTA (Dio-NpHR injection and fiber implant over the VTA of DAT::Cre males). m, Real-time place aversion assay, with constant 7 mW green light delivery on the stimulation side of the chamber. Preference for the non-stimulation side of a chamber in mice expressing or not expressing inhibitory opsin, mean±SEM plotted. No stimulation was delivered in the baseline condition. n, Schematic for closed-loop, attack-triggered inhibition during defeat. o, In attack-triggered inhibition, distribution of the amount of defeat time in which light was delivered (mean is 7.93% of the defeat session). p, Density map of where in t-SNE behavior space animals were when receiving inhibition. q, Difference in occupancy of t-SNE clusters between inhibition (N = 12 males) and no opsin control (N = 10 males) groups (same mice in r–x), measured by individual clusters’ chi2 statistics; black bold numbers label behaviors differentially expressed by resilient and susceptible mice in our observational study. r, Average time across 10 days of defeat being attacked (2-sided -Test, attack-triggered inhibition vs no opsin control, t = −3.03, p = 0.0066). s, Average time across 10 days of defeat fleeing (t = −3.92, p = 8.5E-4). t, Social interaction (SI) time in the SI test (2-sided t-Test, difference in SI time between inhibition and no opsin control groups: 2-sided t-Test, t = 0.04, p = 0.96). u, Average distance between stressed and aggressor mice during the first 5 min of the barrier-separated sensory period across the 10 days of defeat (2-sided Levene test for difference in variance, W = 4.92, p = 0.038). v, Number of entries into the open arms of an elevated plus maze (2-sided t-Test, difference in mean entries between inhibition and no opsin control, t = −0.64, p = 0.53). w, Number of crossings between chambers of 2-chamber arena (2-sided t-Test, difference in mean crossings between inhibition and no opsin control groups: t = −0.024, p = 0.98). x, Percent of time immobile (speed < 1 cm/s) during exploration of a 2-chamber arena (2-sided t-Test, difference in mean immobility between inhibition and no opsin control groups: t = −0.47, p = 0.64). In r–x, mean±SEM plotted. Across panels, *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 10 Difference in NAc(DAT::GCaMP6f) after versus during direct contact phase of defeat is higher in susceptible compared to resilient individuals.
a, Schematic showing phases of defeat stress during and after direct contact. b, Example traces of NAc(DAT::GCaMP6f) during (left) and after (right) the direct contact phase of defeat from day 10 in an example resilient mouse (top) and susceptible mouse (bottom). Transients represented by red dots. Area under the curve (AOC) in next panels refers to the integral between the half max surrounding each transient. c, Difference in NAc(DAT::GCaMP6f) after vs during the contact phase of defeat, mean±SEM plotted (Mixed linear model for difference in AOC by day, resilience category, and their interaction; interaction effect N = 19 males, Z = −2.037, p = 0.042; 2-sided t-Test for difference in ratio of activity in susceptible vs resilient individuals, day 8: t = −3.607, p = 0.02 after Bonferroni correction for 10 tests across 10 days, *p < 0.05). d, Difference in TS(DAT::GCaMP6f) after vs during the direct contact phase of defeat, mean±SEM plotted (Mixed linear model for difference in AOC by day, resilience category, and their interaction; no significant effects).
Supplementary information
Supplementary Tables 1–30
Supplementary Tables 1–29 contain details of the tests performed for determining statistical significance. Supplementary Table 30 outlines the anatomical locations (Paxinos Atlas common reference frame) of the optical fibre tips in mice that underwent fibre photometry.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Willmore, L., Cameron, C., Yang, J. et al. Behavioural and dopaminergic signatures of resilience. Nature 611, 124–132 (2022). https://doi.org/10.1038/s41586-022-05328-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05328-2
This article is cited by
-
Hippocampal area CA2 activity supports social investigation following an acute social stress
Molecular Psychiatry (2025)
-
Prefrontal corticotropin-releasing factor promotes resilience to social stress
Neuropsychopharmacology (2025)
-
Identifying dysfunctional cell types and circuits in animal models for psychiatric disorders with calcium imaging
Neuropsychopharmacology (2025)
-
Resting-state fMRI reveals altered functional connectivity associated with resilience and susceptibility to chronic social defeat stress in mouse brain
Molecular Psychiatry (2025)
-
Recovery of centralities in medial prefrontal and sensory-related cortices associated with social behavior improvements in an autism mouse model
Scientific Reports (2025)