Abstract
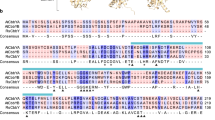
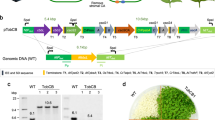
Previous studies have demonstrated that the independent stimulation of either electron transport or RuBP regeneration can increase the rate of photosynthetic carbon assimilation and plant biomass. In this paper, we present evidence that a multigene approach to simultaneously manipulate these two processes provides a further stimulation of photosynthesis. We report on the introduction of the cyanobacterial bifunctional enzyme fructose-1,6-bisphosphatase/sedoheptulose-1,7-bisphosphatase or the overexpression of the plant enzyme sedoheptulose-1,7-bisphosphatase, together with the expression of the red algal protein cytochrome c6, and show that a further increase in biomass accumulation under both glasshouse and field conditions can be achieved. Furthermore, we provide evidence that the stimulation of both electron transport and RuBP regeneration can lead to enhanced intrinsic water-use efficiency under field conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study, the plant transformation constructs and the seed are available from the corresponding authors on reasonable request.
References
Zhu, X. G., Long, S. P. & Ort, D. R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261 (2010).
Simkin, A. J., Lopez-Calcagno, P. E. & Raines, C. A. Feeding the world: improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 70, 1119–1140 (2019).
Simkin, A. J. Genetic engineering for global food security: photosynthesis and biofortification. Plants 8, 586–615 (2019).
Miyagawa, Y., Tamoi, M. & Shigeoka, S. Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat. Biotechnol. 19, 965–969 (2001).
Lefebvre, S. et al. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 138, 451–460 (2005).
Raines, C. A. Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant Cell Environ. 29, 331–339 (2006).
Rosenthal, D. M. et al. Over-expressing the C3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biol. 11, 123 (2011).
Simkin, A. J., McAusland, L., Headland, L. R., Lawson, T. & Raines, C. A. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 66, 4075–4090 (2015).
Simkin, A. J. et al. Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol. J. 15, 805–816 (2017).
Zhu, X. G., de Sturler, E. & Long, S. P. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 145, 513–526 (2007).
Long, S. P., Zhu, X. G., Naidu, S. L. & Ort, D. R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29, 315–330 (2006).
Poolman, M. G., Fell, D. A. & Thomas, S. Modelling photosynthesis and its control. J. Exp. Bot. 51, 319–328 (2000).
Raines, C. A. The Calvin cycle revisited. Photosynth. Res. 75, 1–10 (2003).
Uematsu, K., Suzuki, N., Iwamae, T., Inui, M. & Yukawa, H. Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 63, 3001–3009 (2012).
Ding, F., Wang, M. L., Zhang, S. X. & Ai, X. Z. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 6, 32741 (2016).
Driever, S. M. et al. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Phil. Trans. R. Soc. B 372, 1730 (2017).
Tamoi, M., Nagaoka, M., Miyagawa, Y. & Shigeoka, S. Contribution of fructose-1,6-bisphosphatase and sedoheptulose-1,7-bisphosphatase to the photosynthetic rate and carbon flow in the Calvin cycle in transgenic plants. Plant Cell Physiol. 47, 380–390 (2006).
Ichikawa, Y. et al. Generation of transplastomic lettuce with enhanced growth and high yield. GM Crops 1, 322–326 (2010).
Kohler, I. H. et al. Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. J. Exp. Bot. 68, 715–726 (2017).
Simkin, A. J., McAusland, L., Lawson, T. & Raines, C. A. Overexpression of the Rieske FeS protein increases electron transport rates and biomass yield. Plant Physiol. 175, 134–145 (2017).
Ermakova, M., Lopez-Calcagno, P. E., Raines, C. A., Furbank, R. T. & von Caemmerer, S. Overexpression of the Rieske FeS protein of the cytochrome b6f complex increases C4 photosynthesis in Setaria viridis. Commun. Biol. 2, 314 (2019).
Chida, H. et al. Expression of the algal cytochrome c6 gene in Arabidopsis enhances photosynthesis and growth. Plant Cell Physiol. 48, 948–957 (2007).
Yadav, S. K., Khatri, K., Rathore, M. S. & Jha, B. Introgression of UfCyt c6, a thylakoid lumen protein from a green seaweed Ulva fasciata Delile enhanced photosynthesis and growth in tobacco. Mol. Biol. Rep. 45, 1745–1758 (2018).
Merchant, S. & Bogorad, L. The Cu(II)-repressible plastidic cytochrome c: cloning and sequence of a complementary DNA for the pre-apoprotein. J. Biol. Chem. 262, 9062–9067 (1987).
De la Rosa, M. A., Molina-Heredia, F. P., Hervás, M. & Navarro, J. A. in Photosystem I: Advances in Photosynthesis and Respiration Vol. 24 (ed. Golbeck, J. H.) 683–696 (Springer, 2006).
Finazzi, G., Sommer, F. & Hippler, M. Release of oxidized plastocyanin from photosystem I limits electron transfer between photosystem I and cytochrome b6f complex in vivo. Proc. Natl Acad. Sci. USA 102, 7031–7036 (2005).
Gong, H. Y. et al. Transgenic rice expressing Ictb and FBP/SBPase derived from cyanobacteria exhibits enhanced photosynthesis and mesophyll conductance to CO2. PLoS ONE 10, e0140928 (2015).
Wullschleger, S. D. Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 44, 907–920 (1993).
Pesaresi, P. et al. Mutants, overexpressors, and interactors of Arabidopsis plastocyanin isoforms: revised roles of plastocyanin in photosynthetic electron flow and thylakoid redox state. Mol. Plant 2, 236–248 (2009).
López-Calcagno, P. E. et al. Overexpressing the H‐protein of the glycine cleavage system increases biomass yield in glasshouse and field‐grown transgenic tobacco plants. Plant Biotechnol. J. 17, 141–151 (2018).
Glowacka, K. et al. Photosystem II subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 9, 868 (2018).
Busch, F. A. Opinion: the red-light response of stomatal movement is sensed by the redox state of the photosynthetic electron transport chain. Photosynth. Res. 119, 131–140 (2014).
Engler, C., Gruetzner, R., Kandzia, R. & Marillonnet, S. Golden Gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS ONE 4, e5553 (2009).
Engler, C., Kandzia, R. & Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3, e3647 (2008).
Nakagawa, T. et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34–41 (2007).
Simkin, A. J. et al. Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 85, 36–43 (2013).
Richins, R. D., Scholthof, H. B. & Shepherd, R. J. Sequence of figwort mosaic-virus DNA (Caulimovirus group). Nucleic Acids Res. 15, 8451–8466 (1987).
Horsch, R. B., Rogers, S. G. & Fraley, R. T. Transgenic plants—technology and applications. Abstr. Pap. Am. Chem. S. 190, 67 (1985).
Hoagland, D. R. & Arnon, D. I. The Water-Culture Method for Growing Plants without Soil (College of Agriculture, 1950).
Kromdijk, J. et al. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861 (2016).
Lopez-Calcagno, P. E., Abuzaid, A. O., Lawson, T. & Raines, C. A. Arabidopsis CP12 mutants have reduced levels of phosphoribulokinase and impaired function of the Calvin–Benson cycle. J. Exp. Bot. 68, 2285–2298 (2017).
Dunford, R. P., Catley, M. A., Raines, C. A., Lloyd, J. C. & Dyer, T. A. Purification of active chloroplast sedoheptulose-1,7-bisphosphatase expressed in Escherichia coli. Protein Expr. Purif. 14, 139–145 (1998).
Henkes, S., Sonnewald, U., Badur, R., Flachmann, R. & Stitt, M. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell 13, 535–551 (2001).
Khozaei, M. et al. Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 27, 432–447 (2015).
Hiyama, T. Isolation of photosystem I particles from spinach. Methods Mol. Biol. 274, 11–17 (2004).
Zhao, Y. L. et al. Downregulation of transketolase activity is related to inhibition of hippocampal progenitor cell proliferation induced by thiamine deficiency. Biomed. Res. Int. 2014, 572915 (2014).
Barbagallo, R. P., Oxborough, K., Pallett, K. E. & Baker, N. R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 132, 485–493 (2003).
von Caemmerer, S. et al. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J. Exp. Bot. 55, 1157–1166 (2004).
Baker, N. R., Oxborough, K., Lawson, T. & Morison, J. I. L. High resolution imaging of photosynthetic activities of tissues, cells and chloroplasts in leaves. J. Exp. Bot. 52, 615–621 (2001).
Oxborough, K. & Baker, N. R. An evaluation of the potential triggers of photoinactivation of photosystem II in the context of a Stern–Volmer model for downregulation and the reversible radical pair equilibrium model. Phil. Trans. R. Soc. Lond. B 355, 1489–1498 (2000).
Lawson, T., Lefebvre, S., Baker, N. R., Morison, J. I. L. & Raines, C. A. Reductions in mesophyll and guard cell photosynthesis impact on the control of stomatal responses to light and CO2. J. Exp. Bot. 59, 3609–3619 (2008).
Farquhar, G., von Caemmerer, S. V. & Berry, J. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Sharkey, T. D., Bernacchi, C. J., Farquhar, G. D. & Singsaas, E. L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 30, 1035–1040 (2007).
Vialet-Chabrand, S., Matthews, J. S. A., Simkin, A. J., Raines, C. A. & Lawson, T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 173, 2163–2179 (2017).
von Caemmerer, S. & Farquhar, G. D. Some relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta 153, 376–387 (1981).
Acknowledgements
This study was supported by the Realising Improved Photosynthetic Efficiency (RIPE) initiative awarded to C.A.R. by the University of Illinois. RIPE was possible through support from the Bill & Melinda Gates Foundation, DFID and FFAR, grant no. OPP1172157. This work was also supported by the Biotechnology and Biological Sciences Research Council (BBSRC) grant no. BB/J004138/1. We thank J. Matthews (University of Essex) for help with the data analysis, E. A. Pelech (University of Illinois) and S. Subramaniam (University of Essex) for help with plant growth, P. A. Davey (University of Essex) and R. Gossen (University of Helsinki) for help with gas exchange, and D. Drag, B. Harbaugh and the Ort lab (University of Illinois) for support with the field trials.
Author information
Authors and Affiliations
Contributions
P.E.L.-C. and A.J.S. generated the transgenic plants. P.E.L.-C., A.J.S., K.L.B. and S.J.F. performed the molecular and biochemical experiments. P.E.L.-C., A.J.S. and K.L.B. carried out the plant phenotypic and growth analysis and performed the gas-exchange measurements. S.V.-C. made the measurements of photosynthesis during light induction. A.J.S. and S.J.F. performed the enzyme assays on selected lines. All authors carried out data analysis on their respective contributions. C.A.R. and T.L. designed and supervised the research. P.E.L.-C., A.J.S. and C.A.R. wrote the manuscript. T.L. contributed to the editing of the manuscript and finalizing of the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15 and Table 1.
Rights and permissions
About this article
Cite this article
López-Calcagno, P.E., Brown, K.L., Simkin, A.J. et al. Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nat. Plants 6, 1054–1063 (2020). https://doi.org/10.1038/s41477-020-0740-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0740-1
This article is cited by
-
Structure, regulation and assembly of the photosynthetic electron transport chain
Nature Reviews Molecular Cell Biology (2025)
-
Integration of metabolite and transcriptome profiles of cultivated and wild rice to unveil gene regulatory networks and key genes determining rice source and sink strength
Functional & Integrative Genomics (2025)
-
Effect of different Bradyrhizobium japonicum inoculants on physiological and agronomic traits of soybean (Glycine max (L.) Merr.) associated with different expression of nodulation genes
BMC Plant Biology (2024)
-
Comparative transcriptome and coexpression network analysis revealed the regulatory mechanism of Astragalus cicer L. in response to salt stress
BMC Plant Biology (2024)
-
From leaf to multiscale models of photosynthesis: applications and challenges for crop improvement
Photosynthesis Research (2024)